ISSA Proceedings 2010 – Argumentative Insights For The Analysis Of Direct-To-Consumer Advertising
No comments yet 1. Introduction
1. Introduction
Among the scholars interested in direct-to-consumer advertising (DTCA), there is more and more interest in examining argumentation in this particular type of ads. On the one hand, the argumentative nature of direct-to-consumer advertising can hardly be overlooked,[i] but on the other hand, this argumentative nature is also often the main source of criticism that the opponents of DTCA advance. Critics often point out that direct-to-consumer advertising, as the name suggests, is a promotional activity that aims at increasing the sales of the medicine advertised (Chandra & Holt, 1999; Gilbody, Wilson & Watt, 2005; Mintzes, 1998; Wolfe, 2002), rather than a source of information that raises the health literacy of the public and allows patients to be more involved in their healthcare, as DTCA supporters claim (Auton, 2004, 2007; Calfee, 2002; Jones & Garlick, 2003). In a previous paper (Mohammed & Schulz, 2010), we have argued that the argumentative nature of DTCA is not necessarily what diminishes its educational potential. Ideally, it is possible for direct-to-consumer advertising to fulfil both educational and promotional purposes.
Reasonable argumentation can reconcile the promotional and educational aims of direct-to-consumer advertising. A reasonable defence of this claim will react to the doubt of patients as well as to the competing claims and arguments of other pharmaceutical companies. Such a defence will provide assistance for the patients in making well-informed decisions and if successful will also convince them to ask their doctors to prescribe medicine x for them. The latter is the heart of pharmaceuticals’ promotional interest. However, our previous analysis of strategic manoeuvring in DTC ads suggests that pharmaceutical companies are more interested in getting the claim that promotes their medicine accepted by an audience of consumers rather than by an audience of patients who would like to be more involved in their health care. That is mainly reflected by the choice of relying significantly on arguments that promote the medicine on the basis of qualities that relate to its non-medical attributes (in our earlier study, we have referred to such arguments, which address the non-medical attributes of a medicine, such as the ease of use of a medicine, its cost benefits, and social-psychological enhancements attributes … etc, as convenience appeals). Such a choice reflects an interest in convincing a potential consumer who would certainly care about what is convenient, rather than convincing an active patient who is more concerned with the effectiveness and safety of his treatment option. Even though the findings of our analysis are in line with a significant part of the criticism of the practice of direct-to-consumer advertising, a test of the generalisability of such findings seems to be necessary.
One of the most common methodologies of testing the generalisability of empirical claims about discourse is the method of content analysis. Quantitative content analysis is a standard methodology in the social sciences for studying, structuring and analysing the content of communication. It is an effective, systematic, and replicable data reduction technique that helps compressing many words of text or images into fewer content categories based on explicit rules of coding, and it has the appealing feature of being useful in dealing with big volumes of data. In spite of the increasing awareness of the central role that argumentation plays in DTC advertising, argumentative considerations have not yet been adequately incorporated into the content analysis of DTCA. Existing coding schemes are not refined enough to capture argumentative characteristics of direct-to-consumer ads. Most content analysis in the field of DTCA are used to depict the variety of information that had been delivered in the ads without paying attention to the argumentative structure that links the different statements in the ads.
In this paper, we aim at discussing the possibility of designing a coding scheme to be used in a content analysis study that tests the generalisability of our empirical claims about DTCA. We shall first, in section 2, discuss the state of the art in the study of DTCA from the perspective of content analysis. This is intended to highlight methodological characteristics of content analysis in the particular area of DTCA. In view of the discussion, we shall, in section 3, develop a proposal for a coding scheme that tests the generalisability of our claim. In section 4, we will discuss, briefly, the challenges that face our proposal.
2. The state of the art
One of the most important content analysis of DTC ads, in which the researchers were immediately concerned with the argumentation used in DTCA, was conducted by Robert Bell, Richard Kravitz and Michael Wilkes from the Department of Communication, University of California, USA (Bell et al., 2000). Bell et al. analysed DTC ads of prescription drugs appearing in 18 consumer magazines from 1989 through 1998 (a total of 320 distinct ads representing 101 brands and 14 medical conditions). Their aim was to explore trends in prevalence, shifts in the medical conditions for which drugs are promoted, reliance on financial and nonmonetary inducements, and appeals used to attract public interest.
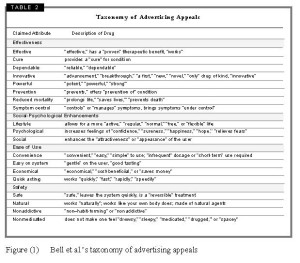
In order to document the advertising appeals used to enhance a patient’s interest in the drugs, each ad was coded for the presence or absence of 42 keywords (adjectives, adjectival phrases, or adverbs that reflect claims about the drug’s nature or impact). Each advertisement was coded for the use of these descriptors to depict the medicine advertised. After coding for the presence or absence of these terms and phrases, related terms were grouped in (19) categories of product attributes. So for example, terms like “advancement,” “breakthrough,” “a first,” the “only” drug of kind, “innovative,” “novel,” and “new” were grouped in the attribute category “Innovative”. These categories were further grouped in four main ‘types’ of appeals: effectiveness, social-psychological benefits, ease of use, and safety. Effectiveness appeals included attributes such as effective, cure, dependable, innovative, powerful, prevention, reduced mortality or symptom control. Social-Psychological appeals included attributes that relate to lifestyle, psychological benefits or social enhancements. Ease of use appeals included attributes such as convenience, easy on system, economical or quick acting. Finally, safety appeals included attributes such as safe, natural, non-addictive or non-medicated (see Figure (1) below).[ii]
Bell et al.’s taxonomy has been used by a number of more recent content analysis of DTC ads, such as the study of Wendy Macias and Liza Stavchansky Lewis, who examined the content and form of 90 DTC drug Web sites (Macias & Lewis, 2003)[iii] and by researchers at Dana-Farber Cancer Institute in Boston, who examined 75 DTC ads for oncology drugs (15 distinct ads) that appeared in three cancer patient-focused magazines, CURE, Coping with Cancer and MAMM, in 2005 (Abel et al., 2007).[iv]
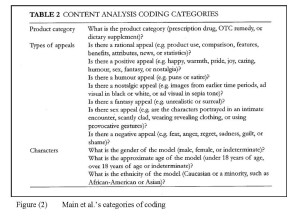
Another influential content analysis study of DTC ads is that of Kelly Main, Jennifer Argo and Bruce Huhmann, who were interested in identifying the kind of information and /or appeals that are being provided to consumers in DTC ads (Main et al., 2004). Main et al. devised their own taxonomy of advertising appeals when studying the ads that appeared in the December issues of 1998, 1999 and 2000 in 30 US magazines (a total of 365 ads). The taxonomy distinguished between rational appeals, positive emotional appeals and negative emotional appeals, and further distinguished between four main subtypes of positive emotional appeals: humour, nostalgic, fantasy and sex appeals (see Figure (2) below). A slightly modified version of this taxonomy has been also used by Dominick Frosch, Patrick Krueger, Robert Hornik, Peter Cronholm and Frances Barg from the University of California and the University of Pennsylvania, who examined how television DTC ads attempt to influence consumers (Frosch et al., 2007).
Another significant contribution to the study of DTC ads using the method of content analysis is the research conducted at by researchers at the institute of Communication and Health at the Università della svizzera italiana in Switzerland. Peter Schulz and Uwe Hartung developed a codebook for analysing DTC ads, aiming to capture and assess relevant argumentative differences between patient-oriented and physician-oriented communication (unpublished manuscript). In particular, it was expected that variations will occur with respect to the use of medical evidence versus the emotional appeal. In order to capture and assess the expected argumentative differences, the researchers included in their corpus also adverts that are directed to physicians. 120 print adverts regarding health conditions published between 2003 and 2006 in two U.S. magazines, namely Time and Good Housekeeping, as well as in two leading medical journals, New England Journal of Medicine and JAMA (Journal of American Medical Association), had been collected. In their codebook, Schulz and Hartung suggest 8 categories of what they refer to as “substance of premise”. The categories are: medicament helps, medicament has no/low side effects, medicament is cheap, medicament is widely used, disease or condition against which the medicament is indicated is bad, medicament is widely studied, use-related premises and fringe benefits (see Figure (3) below).
3. Testing the generalisability of our claims on direct to consumer ads
What we would like to test, by using the method of content analysis, is whether the claim that DTC ads are addressed to an audience of consumers rather than an audience of patients applies in general to DTC ads and is not specific to the particular ads that we analysed in our earlier study. In our earlier analysis, this conclusion was reached on the basis of the central role that convenience appeals played in the ads analysed. For example, in one of the ads, in which Takeda Pharmaceuticals promote their sleeping pills Rozerem, two out of the four main arguments that are used to support the claim that Rozerem is a good treatment against insomnia were convenience appeals. In the ad, Takeda Pharmaceuticals express this claim quite strongly. Rozerem is a sleep aid like no other, they claim (see Rozerem ad below).
In support of this claim, four main arguments are presented: Rozerem is approved for adults having trouble falling asleep (1.1a), Rozerem is the first and only prescription sleep aid that has no potential for abuse or dependence (1.1b), you can take Rozerem when you need it and stop when you don’t (1.1c) and Rozerem makes you dream (1.1d) which one can easily infer from the opening line of the ad, namely that when you can’t sleep, you can’t dream. Argument 1.1b is further supported by reference to clinical studies in which Rozerem shows no potential for abuse or dependence (1.1b.1). The structure of argumentation is illustrated below.
1 Rozerem is a good treatment against insomnia
1.1a Rozerem is approved for adults having trouble falling asleep
1.1b Rozerem is the first and only prescription sleep aid that has no potential for abuse or dependence
1.1b.1 in clinical studies Rozerem shows no potential for abuse or dependence
1.1c you can take Rozerem when you need it and stop when you don’t
1.1d Rozerem makes you dream
What coding variable can we use to reflect the central role that convenience appeals play in a DTC ad? One indicator of such a role is the number of such appeals in the ad. So, maybe even prior to the task of reflecting the central role of convenience appeals is the task of representing the presence of convenience appeals. Convenience appeals, as we used them in our earlier analysis, are arguments that promote the medicine on the basis of qualities that relate to its non-medical advantages. They are in this sense more general than the product attribute of convenience proposed by Bell et al. (2000). Unlike Bell et al.’s category, which refers solely to arguments in which claims about the medicine’s convenience of use is made, our convenience appeals is a type of appeals that covers Bell’s claims about convenience of use as well as other non-medical attributes, such as the medicine’s cost, its enhancement of lifestyle and of the social and psychological being of those who take it … etc. In this sense, our convenience appeals comprise Bell et al.’s both ease of use and social-psychological attributes (i.e. premises about psychological enhancement, lifestyle enhancement, social enhancement, convenience, quick acting, economical and easy on system). This type of appeals has also been represented in the codebook of Schulz and Hartung. A few of the coding categories for the variable “substance of premise” represent what can be considered as a convenience appeal (for example: -11- Medicament helps fast, its effect sets on quickly, -30- Medicament is cheap / its use is economic, -70- Use-related premises such as Medicament is easy to handle, easy to apply, convenient or does not create unpleasant sensations, -71- Medicament is easy to use, easy to apply or that no special abilities are needed to apply it, -72- Medicament has no unpleasant taste or odour, is agreeable for children, -74- Medicament has an easy schedule for taking, or that it has no temporal or situational requirements).
In order to represent the presence of such appeals, a variable needs be designed that describes the type of appeal involved in the argument (a content variable at the premise level). For every premise, coders would have to choose between three main types of appeals: an effectiveness appeal when the premise refers to qualities that relate to the medical effect of the medicine: it controls symptoms, it is powerful, it is long lasting … etc, a safety appeal when the premise refers to qualities that relate to the side effects of the medicine: it is natural, it does not have serious side effects … etc, and a convenience appeal when the premise refers to qualities that relate to the non-medical advantages of the medicine, including the ease of use, economical benefits, quick acting, life style, and social-psychological enhancements … etc. This proposal for a coding scheme is illustrated in Figure (5) below:
The percentage of the number of convenience appeals in relation to the total number of appeals might be an indication of the importance of such appeals. However, this is not always the case. The argumentative role that such appeals play is an important factor to consider, especially when ads employ a complex structure of argumentation.[v] For example, when ads employ argumentation in a subordinative structure, i.e. when some premises support the main claim indirectly by supporting other premises, the percentage of convenience appeals no longer reflects their argumentative importance. The Rozerem ad is an example. The ad includes five premises, one of which (1.1b.1 in clinical studies Rozerem shows no potential for abuse or dependence) supports the main claim about Rozerem by supporting the safety appeal (1.1b Rozerem is the first and only prescription sleep aid that has no potential for abuse or dependence). If one counts the total number of premises, one would think that 40% of the premises (two out of five premises) are convenience appeals, but once the argumentative role is considered one realises that convenience appeals constitute 50% of the premises (two out of four lines of argumentation/ four main arguments employ convenience appeals).
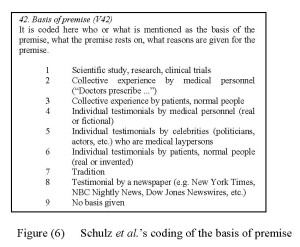
There seems to be a need to represent the argumentative role that a certain premise plays. One way of doing this would be to code premises into main and sub-arguments. While main arguments support the main claim directly, sub-arguments are elaborations that support other arguments and only through such a support lend support to the main claim. This coding variable, which we can call premise role or argument structure would come prior to the coding variable substance of premise discussed earlier. Premises that are coded as main arguments would be further coded according to the variable premise substance discussed earlier, premises that are coded as sub-arguments need a different variable for coding. Something along the line of what Schulz and Hartung refer to as “basis for premise”, in which it is coded who or what is mentioned as the basis of the premise, what the premise rests on, what reasons are given for the premise (See Figure (6) below).
The coding categories used by Schulz and Hartung for the coding variable substance of premise would need to be divided into two coding variables: substance of main arguments and substance of sub-arguments. Variables such as -40- Medicament is widely used, patient preferred it would belong to the latter. This kind of argument is usually presented as a sub-argument in support of main arguments.
4. Discussion
The biggest challenge for our proposal to distinguish between main and sub-arguments is to maintain high inter-coder reliability. This kind of reliability, which refers to the amount of agreement or correspondence among two or more coders, is crucial for the generalisability of our findings. Coding instructions should be clearly formulated to assist the coders in distinguishing between main and sub-arguments, a distinction that is not necessarily easy to make if the coders are not familiar with concepts of argumentation theory. Good inter-coder reliability can be achieved by including indicators for subordinative argumentations as well as examples of this kind of argumentation structure in the coding instructions. Van Eemeren et al.’s Argumentative Indicators in Discourse (2007) can be a good source for such indicators.
NOTES
[i] Several studies, conducted by Rubinelli (2005) and Rubinelli et al. (2006, 2007) among others, have shown that direct-to-consumer ads exhibit clear argumentative features, and that these features are recognised by potential consumers. For example, Rubinelli, Nakamoto, Schulz and De Saussure report that in their pilot study, 71 out of the 72 respondents recognised the argumentative structure of the ads they were shown (2006: p. 339).
[ii] Bell et al. report that, in the ads they analysed, the categories of appeals used most frequently are effective, used in 57% of ads, controls symptoms and innovative, used in 41% of the ads each, and convenience, used in 38% of the ads. The rest of the categories appeared in the following frequencies: prevents condition (16%), nonmedicated (14%), psychological enhancement and safe (each in 11% of the ads), powerful (9%), reduced mortality and natural (each in 7% of the ads), lifestyle enhancement and quick acting (each in 6% of the ads), economical and not addictive (each in 5% of the ads), dependable (4%), cures, easy on system and social enhancement (each in 3% of the ads).
[iii] Macia and Lewis (2003) report that while the advertising appeals used in DTC sites are similar to those found in print ads, DTC sites offer more monetary incentives but provide a much higher degree of medical and drug information. They argue that the latter makes DTC sites better suited to fulfilling Food and Drug Administration (FDA) guidelines.
[iv] Abel et al. report that DTC ads for oncology drugs make more appeal to effectiveness than to safety. The ads are reported to be difficult to read in general but the text outlining the benefits is reported to have the highest readability score. According to Abel et al., even though the amount of text devoted to benefits versus risks and side effects was roughly the same, information on benefits was more prominent: information about benefits appeared in the top third of the advertisement text while descriptions of side effects and risks typically ran in the bottom third, and the largest type size of the text explaining the benefits was about twice as large as the largest text outlining side effects and risks.
[v] We follow the distinction van Eemeren et al. (2002) make between a single structure of argumentation, in which a standpoint is supported by one single argument, and a complex structure of argumentation in which the standpoint is supported by more than one argument. A complex structure of argumentation can be either multiple argumentation, in which the standpoint is supported by more than one alternative defense, coordinative argumentation, in which the standpoint is defended by several arguments taken together, or subbordinaive argumentation, in which the standpoint is supported by arguments that are further supported by other arguments (2002, pp. 63-87).
REFERENCES
Auton, F. (2004). The patient as consumer: the advertising of pharmaceuticals directly to consumers should be allowed and encouraged. Economic Affairs, 27(2), 64-72
Abel, G. A., Lee, S. J. & Weeks, J. C. (2007). Direct-to-Consumer Advertising in Oncology: A Content Analysis of Print Media. Journal of Clinical Oncology, 25(10), 1267-1271
Bell, R. A., Kravitz, R. L. & Wilkes, M. S. (2000). Direct-to-consumer prescription drug advertising, 1989-1998. A content analysis of conditions, targets, inducements, and appeals. The Journal of family practice, 49(4), 329-35.
Calfee, J.E. (2002). Public policy issues in direct-to-consumer advertising of prescription drugs. Journal of Public Policy and Marketing, 21(Fall), 174-194.
Chandra, A. , & Holt, G. A. (1999). Pharmaceutical advertisements: how they deceive patients. Journal of Business Ethics, 18(4), 359-366.
Eemeren, F. H. van, Grootendorst, R., & Snoeck Henkemans, A. F. (2002). Argumentation: Analysis, Evaluation, Presentation. Mahwah, New Jersy: Lawrence Erlbaum.
Eemeren, F. H. van, Houtlosser, P., & Snoeck Henkemans, A. F. (2007). Argumentative Indicators in Discourse: A Pragma-Dialectical Study. Dordrecht: Springer Netherlands.
Frosch, D. L., Kruger, P. M., Hornik, R. C., Cronholm, P. F., & Barg, F. K. (2007). Creating demand for prescription drugs: A content analysis of television direct-to-consumer advertising. Annals of Family Medicine, 5, 6-13.
Gilbody, SM, Wilson, P, Watt, IS. (2005) The benefits and harms of direct to consumer advertising: a systematic review. Quality and Safety in Health Care, 14, 246-250.
Jones, T. & Garlick, W. (2003). Should drug companies be allowed to talk directly to patients? British Medical Journal 326, 1302-1303.
Macias, W. & Lewis, L.S. (2003). A Content Analysis of Direct-to-Consumer (DTC) Prescription Drug Web Sites. Journal of Advertising, 32(4), 43-56
Main, K.J., Argo, J.J. and Huhmann, B.A. (2004). Pharmaceutical advertising in the USA: information or influence?. International Journal of Advertising, 23(1), 119-42.
Meyers, R., D. R. Seibold, and D. Brashers. 1991. Argument in Initial Group Decision-Making Discussions: Refinement of a Coding Scheme and a Descriptive Quantitative Analysis. Western Journal of Communication, 55(1), 47-68.
Mohammed, D., & Schulz, P.J. (2010). Audience Adaptation in Direct-to-Consumer Advertising. A paper presented at the 13th Biennial Wake Forest Argumentation Conference. Wake Forest University, Winston-Salem, NC, USA. 19-21 March 2010.
Rubinelli, S. (2005). ‘Ask your doctor’. Argumentation in advertising of prescription medicines. Studies in Communication Sciences. Special Issue on Health Literacy, 5, 75-98.
Rubinelli, S., Nakamoto, K., Schulz, P.J. & de Saussure, L. (2006). What are we to think about consumer advertising? A case-study in the field of misinterpreted argumentation. Studies in Communication Sciences, 6(2), 337-348.
Rubinelli, S., Nakamoto, K. & Schulz, P.J. (2007). Reading direct-to-consumer advertising. A qualitative study from argumentation theory on its dialectical and rhetorical features. In F. H. van Eemeren, B. J. Garssen, J. A. Blair and Ch. A. Willard (Eds.), Proceedings of the 6th Conference of the International Society for the Study of Argumentation (pp. 1211-1217). Amsterdam: Sic Sat.
Schulz, P. J., & Hartung, U. (unpublished manuscript). Analysing prescription medication advertising directed at consumers (DTCA).
Wolfe, S. M. (2002). Direct-to-consumer advertising – education or emotion promotion? New England Journal of Medicine, 346, 524 -526.
You May Also Like
Comments
Leave a Reply